FOCUS ON CLINICAL
EVIDENCE
OGIVRI has achieved all the benchmarks for biosimilarity in
efficacy and safety to Herceptin®.1-3
EFFICACY
Study design1
The HERITAGE trial was a confirmatory, double-blind, Phase 3 equivalence trial comparing the efficacy and safety of OGIVRI to Herceptin in 500 adult patients with HER2+ metastatic breast cancer.1

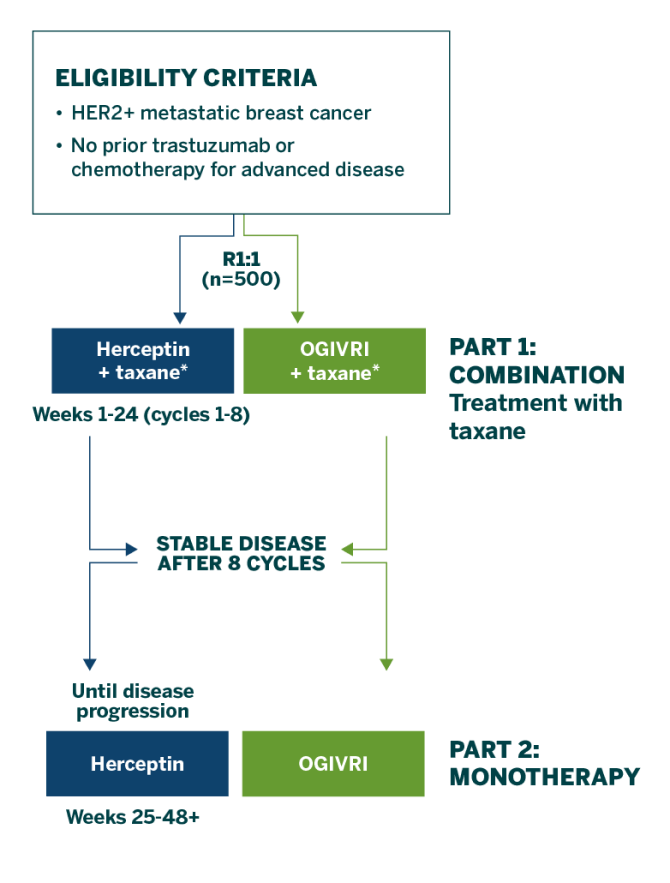
*OGIVRI dosed at 8 mg/kg IV loading dose followed by 6 mg/kg IV Q3W. Docetaxel or paclitaxel selected by physician’s choice.
Study Endpoints
- Primary endpoint: ORR (complete and partial response) at Week 241
- Other endpoints included adverse events, LVEF, and immunogenicity at Week 24 and Week 48, and PFS and OS at Month 361
Baseline Characteristics
- Demographic, disease, and baseline characteristics were similar between treatment groups with no clinically relevant differences observed1
IV=intravenous; LVEF=left ventricular ejection fraction; ORR=overall response rate; OS=overall survival; PFS=progression-free survival; Q3W=every 3 weeks; R=randomized.
ORR at Week 241
- Primary endpoint met: there were no significant differences in ORR at Week 24 between treatment groups1
- ORR ratio (1.09; 90% CI: 0.974-1.211) and ORR difference (5.53; 95% CI: -3.08-14.04) were within the defined equivalence boundaries*1
*ORR ratio 90% CI equivalence boundaries were defined as 0.81 to 1.24 and ORR difference equivalence boundaries were defined as -15% and 15%.
- Primary endpoint met: there were no significant differences in ORR at Week 24 between treatment groups1
- ORR ratio (1.09; 90% CI: 0.974-1.211) and ORR difference (5.53; 95% CI: -3.08-14.04) were within the defined equivalence boundaries*1
*ORR ratio 90% CI equivalence boundaries were defined as 0.81 to 1.24 and ORR difference equivalence boundaries were defined as -15% and 15%.
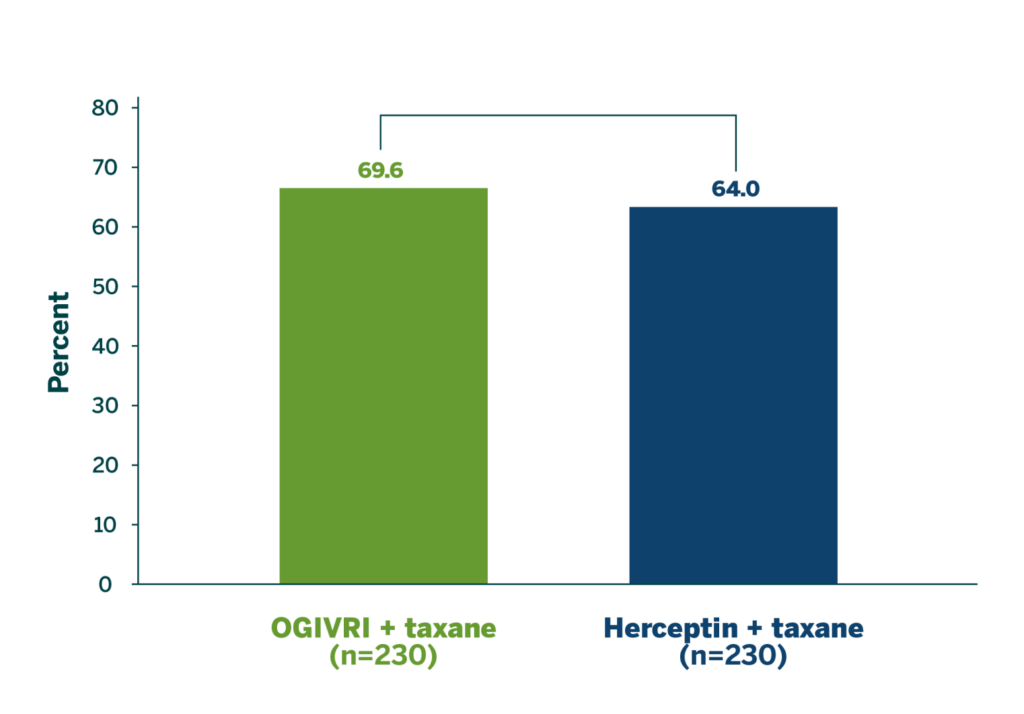
Findings in the Heritage trial are comparable to other recent multicenter studies of trastuzumab + taxane in HER2+ patients.4
CI=confidence interval; ORR=overall response rate.
First FDA-approved Trastuzumab Biosimilar With PFS at 36 Months2
There were no statistically significant differences in PFS at 36 months between treatment groups.2
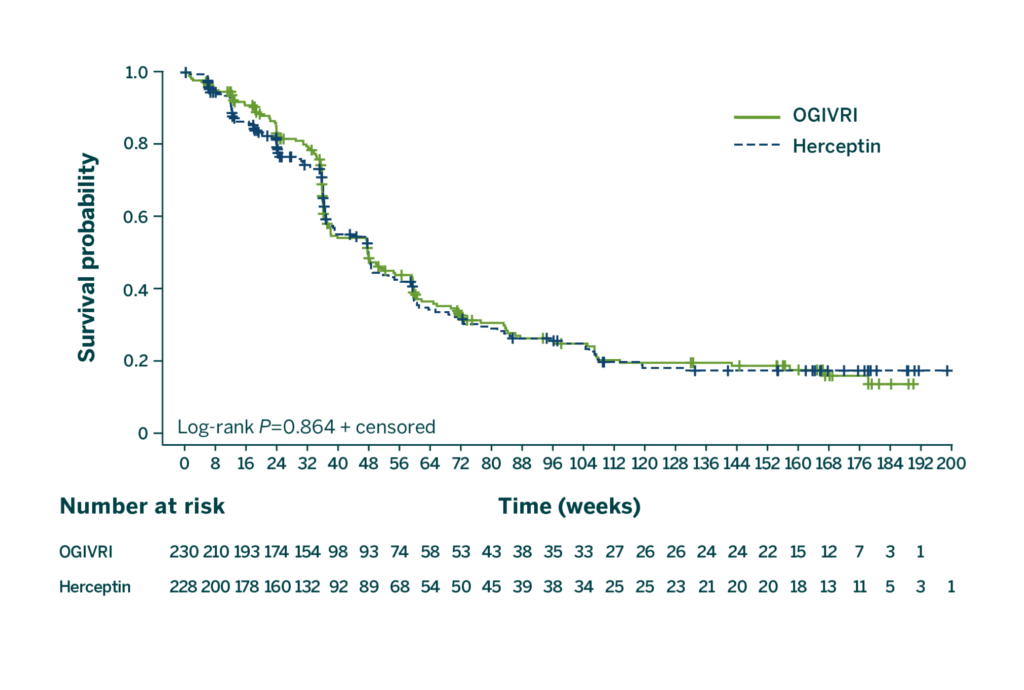
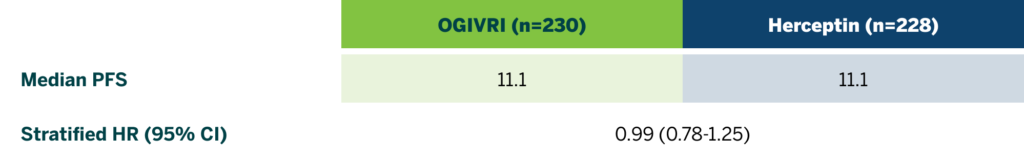
CI=confidence interval; HR=hazard ratio; PFS=progression-free survival.
First FDA-approved Trastuzumab Biosimilar With OS at 36 Months2
There were no statistically significant differences in OS at 36 months between treatment groups.2
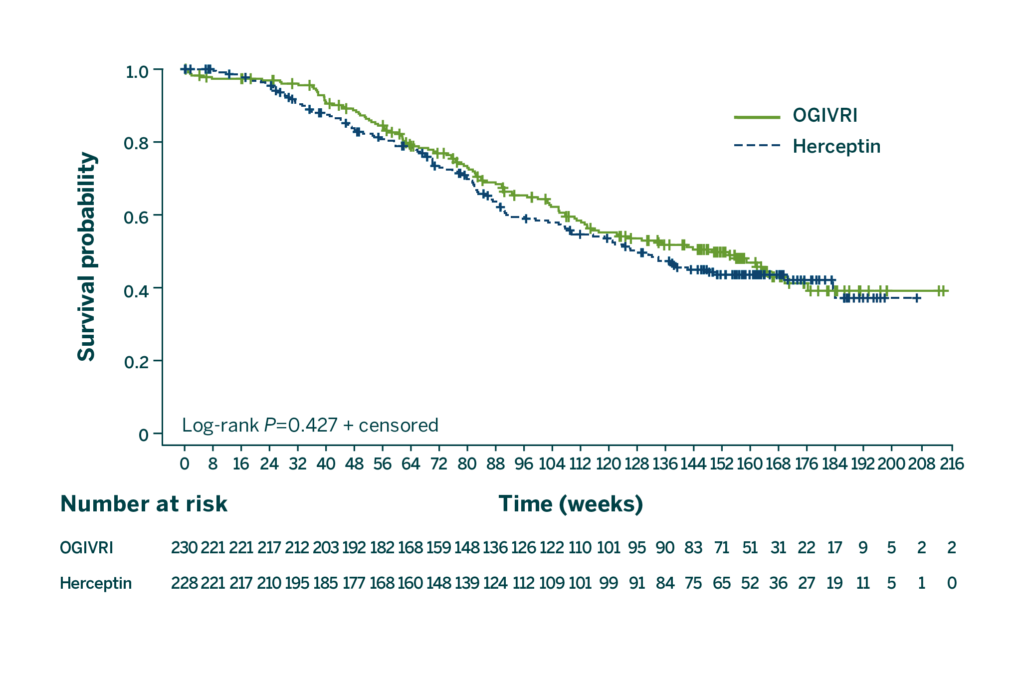
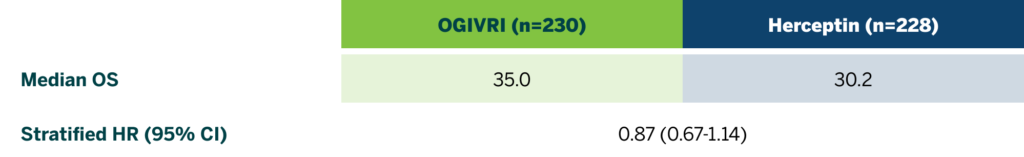
CI=confidence interval; HR=hazard ratio; OS=overall survival.
SAFETY
Safety Data Demonstrate the Biosimilarity1
Rates of treatment-emergent adverse events (TEAEs) were similar between the OGIVRI and Herceptin groups at Week 24.1
All grade TEAEs by Week 24 in the overall safety population (≥ 10% in either group).1
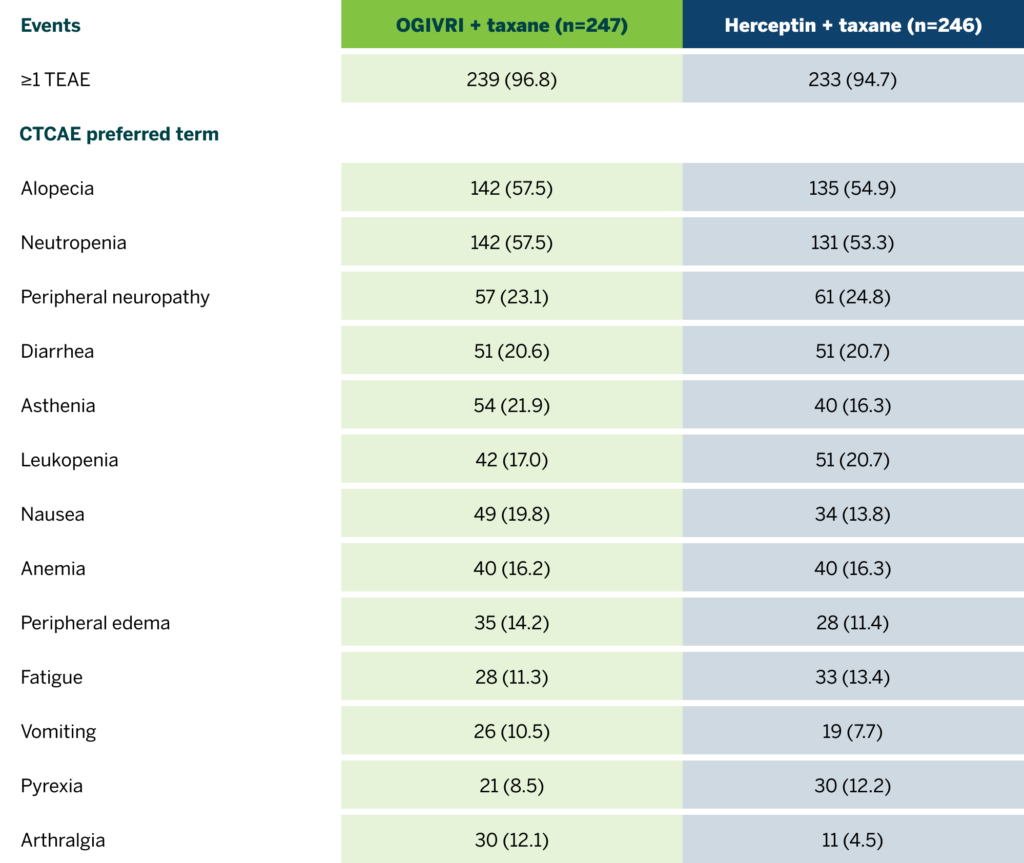
CTCAE=common terminology criteria for adverse event.
No significant Difference in Median LVEF Between OGIVRI and Herceptin1
There was no statistically significant difference in median LVEF between the 2 treatment groups at Week 24 (safety population).1
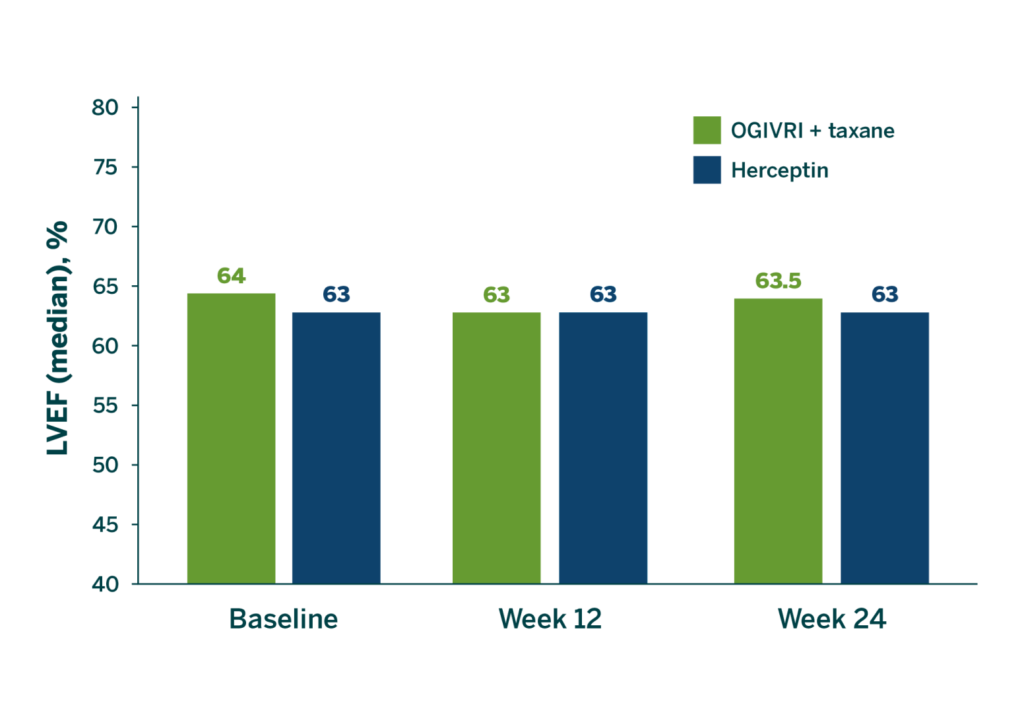
THE OVERALL ANTIDRUG ANTIBODY RATE WAS1:
- 2.4% for OGIVRI + taxane
- 2.8% for Herceptin
LVEF=left ventricular ejection fraction.
No New Safety Signals in Long-term Follow-Up2
There were no statistically significant differences in all grade TEAEs between treatment groups in patients who continued on monotherapy (10% in either group).*2
*Safety data are cumulative from start of monotherapy at Week 24 through 36 months of follow-up from the last patient on study.
TEAE=treatment-emergent adverse event.
REFERENCES
1. Rugo HS, Barve A, Waller CF, et al. Effect of a proposed trastuzumab biosimilar compared with trastuzumab on overall response rate in patients with ERBB2 (HER2)-positive metastatic breast cancer: a randomized clinical trial. JAMA. 2017;317(1):37-47. 2. Rugo HS, Pennella EJ, Gopalakrishnan U, et al. Final overall survival analysis of the phase 3 HERITAGE study demonstrates equivalence of trastuzumab-dkst to trastuzumab in HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2021;188(2):369-377. 3. OGIVRI. Prescribing information. Biocon Biologics Inc; 2024. 4. Burstein HJ, Schrag D. Biosimilar therapy for ERBB2 (HER2)-positive breast cancer: close enough? JAMA. 2017;317(1):30-32.
